Most women spend a third to a half of their lives in perimenopause or postmenopause. Yet most are unsure when it will happen, how long it could last (5–10 years), what the symptoms are, or where to turn for informed care. According to our research with women, this experience can be marked by confusion, quiet suffering, and conflicting recommendations, even from trained providers.
That is starting to shift. Female researchers, founders, clinicians, and public figures are speaking openly about this once-taboo life stage and working toward a better model of care.
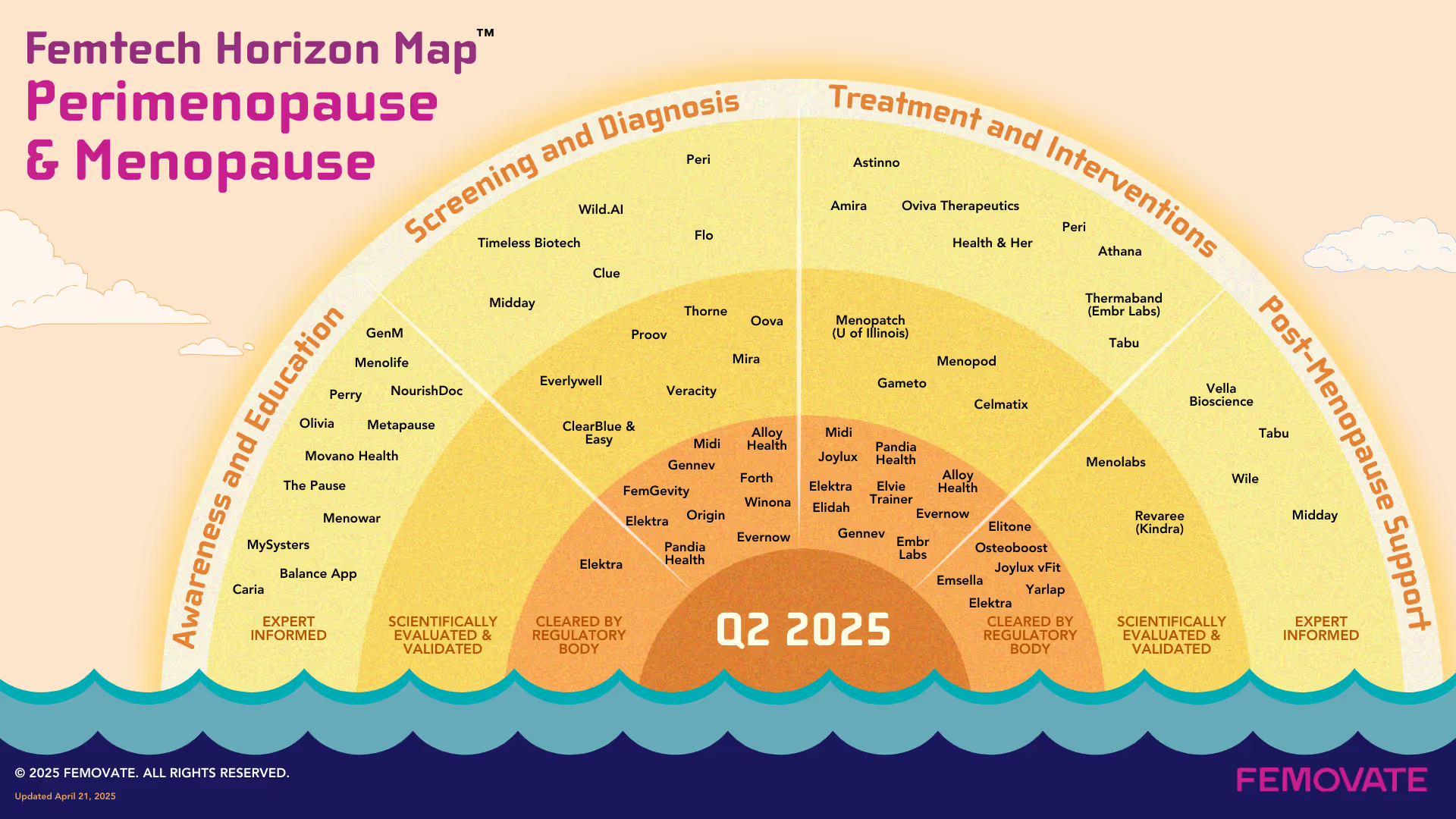
This month's Femtech Horizon Map: Menopause & Perimenopause offers a snapshot of where innovation is happening. It surfaces companies across the continuum of care, from early education and screening to treatment and long-term support, and maps them by level of clinical validation.
The result is not just a product map, but a glimpse of how menopause care is being defined—from the ground up.
After the 2002 Women’s Health Initiative results linked hormone therapy to health risks, menopause care receded across the board. Research slowed. Clinical training pulled back. Product development stalled.
Later analyses clarified what the early headlines missed. The reported risks were largely tied to older women who began treatment well after menopause. For women who start therapy closer to the onset of symptoms, the risk profile is very different. Hormone therapy remains a safe and effective option for many.
With updated guidelines and a new wave of research and startup activity, menopause is back in focus. Innovation is happening across the continuum of care—from education and diagnostics to interventions and long-term management.
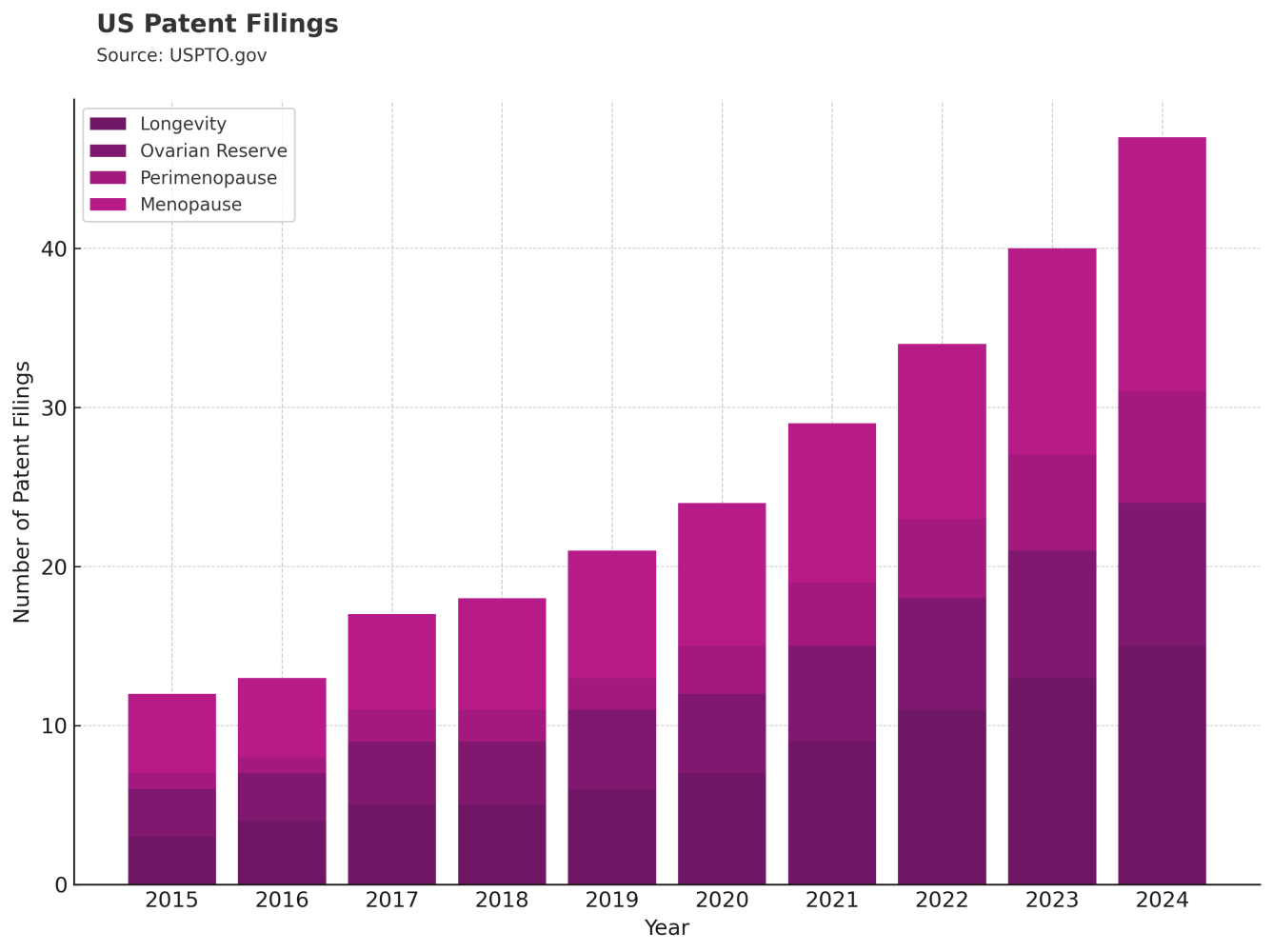
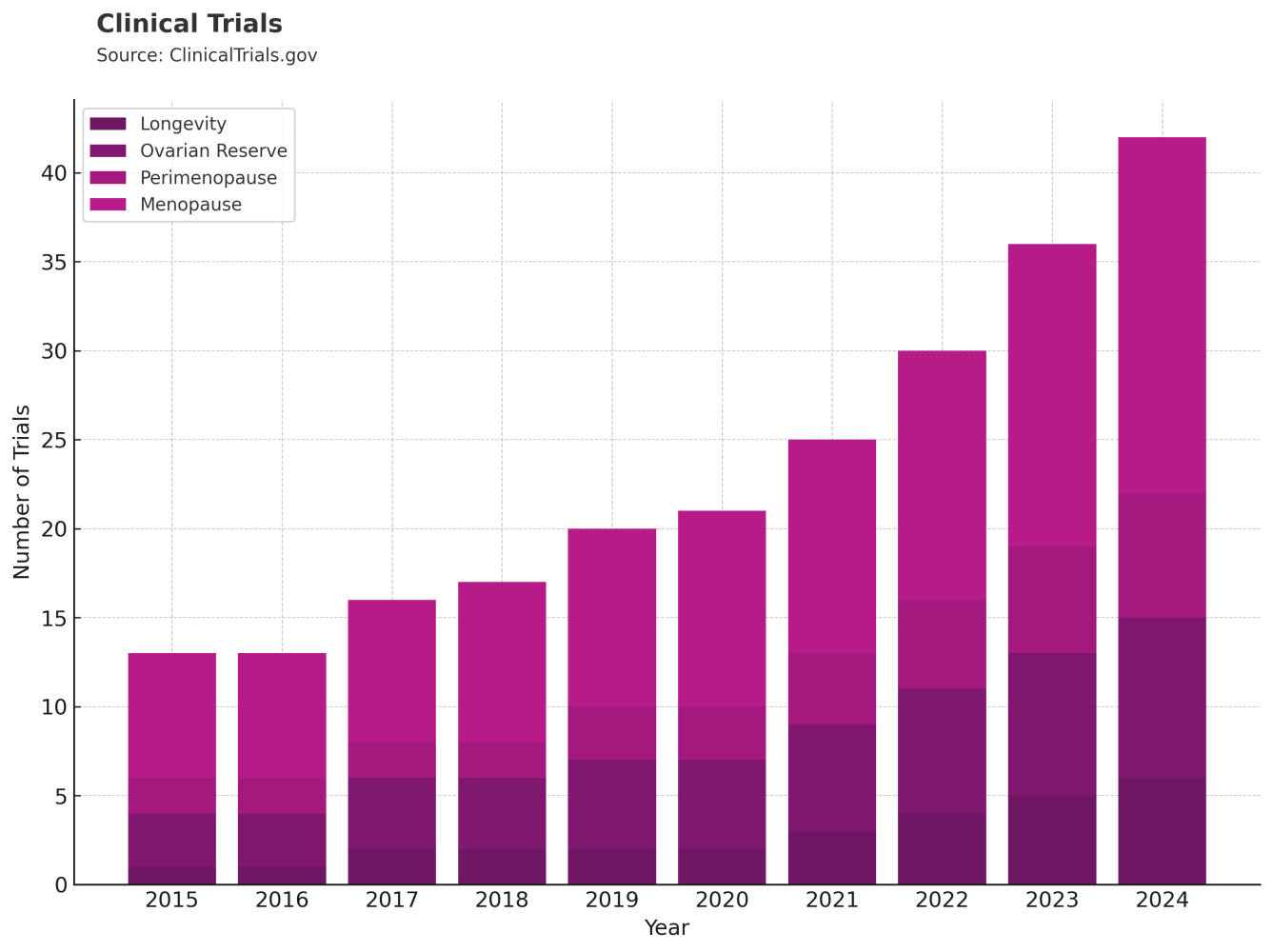
One way we track momentum in a space like this is by looking at what’s being filed and what’s being studied. Patents and clinical trials offer a signal of where research dollars are going and where companies see opportunity.
Data from USPTO.gov and ClinicalTrials.gov show a steady increase in filings and trials related to this space. The trends suggest a growing scientific and commercial interest in redefining midlife health for women.
Awareness & Education Digital platforms like Perry, Caria, and GenM are closing the information gap. They deliver structured, medically reviewed content to help women understand what to expect early in perimenopause. Community-led tools are also being used in workplace benefits and public health campaigns.
Screening & Diagnosis Hormone tracking tools from Proov and MyForm and Oova provide more accessible insight into the perimenopausal transition. Platforms like Wild.AI and Thorne are applying predictive models and biomarker analysis to help women identify trends in hormone patterns earlier and more accurately.
Treatment & Intervention This is where most of the recent activity is concentrated. Companies including Midi Health, Evernow, Alloy, and Pandia Health are rethinking hormone therapy delivery and symptom management through virtual care. Lisa Health uses behavioral science and wearable data to support real-time adjustments in lifestyle and treatment. Meanwhile, companies like Elitone (Elidah), Joylux, and Vella Bioscience are focusing on sexual wellness, pelvic sloor, and non-hormonal therapies with clinical support.
Post-Menopause Support Longer lifespans mean more time spent in post menopause. Companies are now creating more consumer packaged goods (CPGs) to address sleep, mood, metabolic shifts, and the cognitive changes in menopause. And companies like Osteoboost are pioneering medtech specifically for postmenopausal women.
All of the Above Pioneers in the menopause space, Elektra Health, span the entire care continuum and has become the first virtual menopause care provider to accept both Medicare and Medicaid, expanding access to their services for beneficiaries under these plans.
A new category is emerging: companies working not only to manage menopause, but to delay or reprogram it at the cellular level.
Oviva Therapeutics, Celmatix, and Gameto are approaching menopause through the lens of ovarian biology. Their work includes developing therapeutics to preserve ovarian reserve, extend hormone production, and slow reproductive aging. Academic research is moving in parallel. Columbia University’s VIBRANT study is testing rapamycin as a potential way to delay menopause without compromising health or safety.
These efforts are still early stage, but they signal a fundamental shift. The primary goal is to preserve the protective health effects of ovarian hormones. Estrogen plays a critical role in regulating cardiovascular, cognitive, metabolic, and bone health. When hormone levels decline abruptly during menopause, so does protection against heart disease, dementia, osteoporosis, and other chronic conditions. By extending ovarian function, these new interventions aim to prolong healthspan—not only manage symptoms.
Despite the growth in funding and startup activity, there are areas still underserved:
The projected market size for menopause-related solutions is underestimated. Current estimates often focus narrowly on existing treatments and wellness products. But the true opportunity includes entirely new categories of care: universal screening for hormonal changes starting in a woman’s 30s, personalized HRT guided by continuous data, care models that combine clinical support with lifestyle tracking, and targeted products for every stage and symptom of the transition.
As these categories mature and adoption grows, the total addressable market could reach well beyond current projections by 2030. This is not a niche, it is a long-ignored foundation of women’s lifelong health.
In the next phase of development, we are likely to see:
These shifts are on the horizon, follow Femovate to keep up with the innovation.
Femovate works with founders, clinicians, and researchers to develop and commercialize high-impact women’s health solutions. If you are working in menopause, perimenopause, or longevity, we want to help you deliver the next gen experience for women.
Sources: